Thermal and Kinetic Behaviors of Fallen Leaves and Waste Tires Using Thermogravimetric Analysis
11:16 - 25/03/2019
Natural durability of the culturally and historically important timber: Erythrophleum fordii wood against white-rot fungi
Shrinkage and swelling behavior of archaeological waterlogged wood preserved with slightly crosslinked sodium polyacrylate
Natural durability of Erythrophleum fordii Oliver against white rot fungi
Shrinkage and swelling behavior of archaeological waterlogged wood treated with polyacrylic acid resin
Authors
- Shukai Shi - Nanjing Forestry University
- Xiaoyan Zhou - Nanjing Forestry University
- Weimin Chen - Nanjing Forestry University
- Xin Wang - Nanjing Forestry University
- Thiphuong Nguyen - Nanjing Forestry University
- Minzhi Chen - Nanjing Forestry University
Abstract
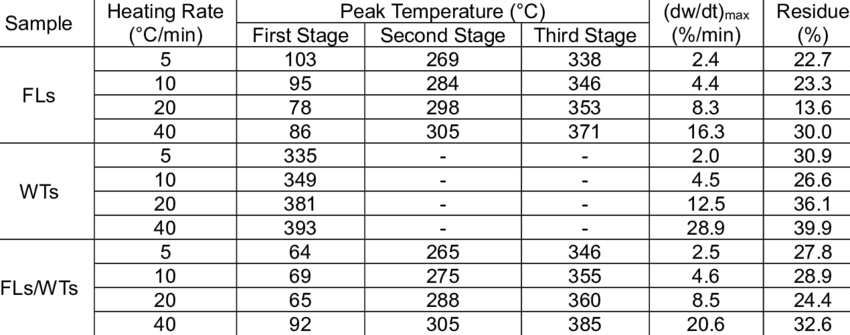
Thermal decomposition characteristics and kinetic parameters of fallen leaves (FLs), waste tires (WTs), and their blends (1:1 weight ratio) were investigated. Pyrolysis experiments were conducted with four different heating rates of 5 °C/min, 10 °C/min, 20 °C/min, and 40 °C/min from 35 °C to 800 °C in a nitrogen atmosphere using a thermogravimetric analysis (TGA). The thermogravimetry/derivative thermogravimetry (TG/DTG) curves indicated that the three samples were mainly degraded in a wide temperature range of 350 °C to 450 °C, and that a greater weight loss rate corresponded to a higher heating rate. An elemental analysis demonstrated that FLs/WTs blends embraced a maximum calorific value that reached 25.24 MJ/kg. Two model-free methods, iso-conversional Kissinger-Akahira-Sunose (KAS) and Flynn-Wall-Ozawa (FWO) were applied on the TGA data of samples to calculate the activation energies. The results showed that the average activation energies of the same feedstock based on KAS and FWO methods were approximately the same, with the highest error within 1.6%. Then, the activation energies obtained were introduced in the Coats/Redfern (CR) model-fitting method to derive the pre-exponential factors, based on first order rate of reaction.


