Thermal Behavior and Kinetic Analysis of Enzymatic Hydrolysis Lignin and High-Density Polyethylene during Co-Pyrolysis
11:05 - 25/03/2019
Natural durability of the culturally and historically important timber: Erythrophleum fordii wood against white-rot fungi
Shrinkage and swelling behavior of archaeological waterlogged wood preserved with slightly crosslinked sodium polyacrylate
Natural durability of Erythrophleum fordii Oliver against white rot fungi
Shrinkage and swelling behavior of archaeological waterlogged wood treated with polyacrylic acid resin
Authors
- Weimin Chen - Nanjing Forestry University
- Xiaoyan Zhou - Nanjing Forestry University
- Shukai Shi - Nanjing Forestry University
- Thiphuong Nguyen - Nanjing Forestry University
- Minzhi Chen - Nanjing Forestry University
Abstract
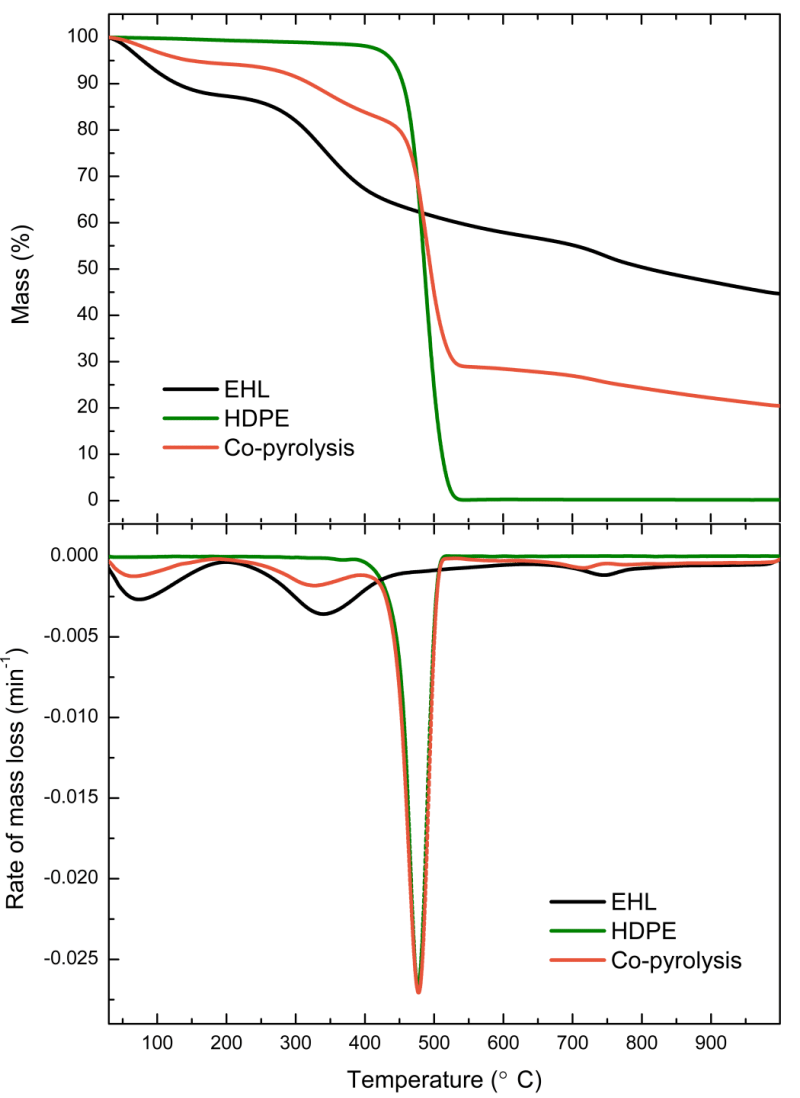
The thermal behaviors of enzymatic hydrolysis lignin (EHL), high-density polyethylene (HDPE), and their blend (50:50 wt.%) were revealed using thermogravimetric analysis coupled with Fourier transform infrared spectroscopy (TG-FTIR). A first-order reaction model (Coats-Redfern) and non-isothermal model-free method (Ozawa-Flynn-Wall) were applied to the TG experimental data to determine the pyrolysis kinetic parameters. The results showed that H2O and CO2 were first released from the EHL due to the degradation of the weakly linked side chains. The degradation of lateral chains, the breakage of aromatic series in the EHL structure, and the β scission of HDPE led to the formation of H2O, CO2, C=O, aromatics, alkanes, and alkenes. Low intensities of H2O, CO2, alkanes, and alkenes were also observed in the final pyrolysis stage due to the degradation of lignin groups. Interactions during co-pyrolysis were observed in the pyrolysis stages of 390 to 542 °C and 563 to 790 °C. The activation energy values of EHL, HDPE, and their blend obtained by the Coats-Redfern method were 48.0 to 94.4 kJ/mol, 230.2 kJ/mol, and 42.7 to 260.1 kJ/mol, respectively. When the Ozawa-Flynn-Wall method was applied, activation energy ranges of 121.4 to 243.7 kJ/mol, 143.5 to 335.9 kJ/mol, and 74.8 to 260.9 kJ/mol for EHL, HDPE, and their blend, respectively, were observed.


